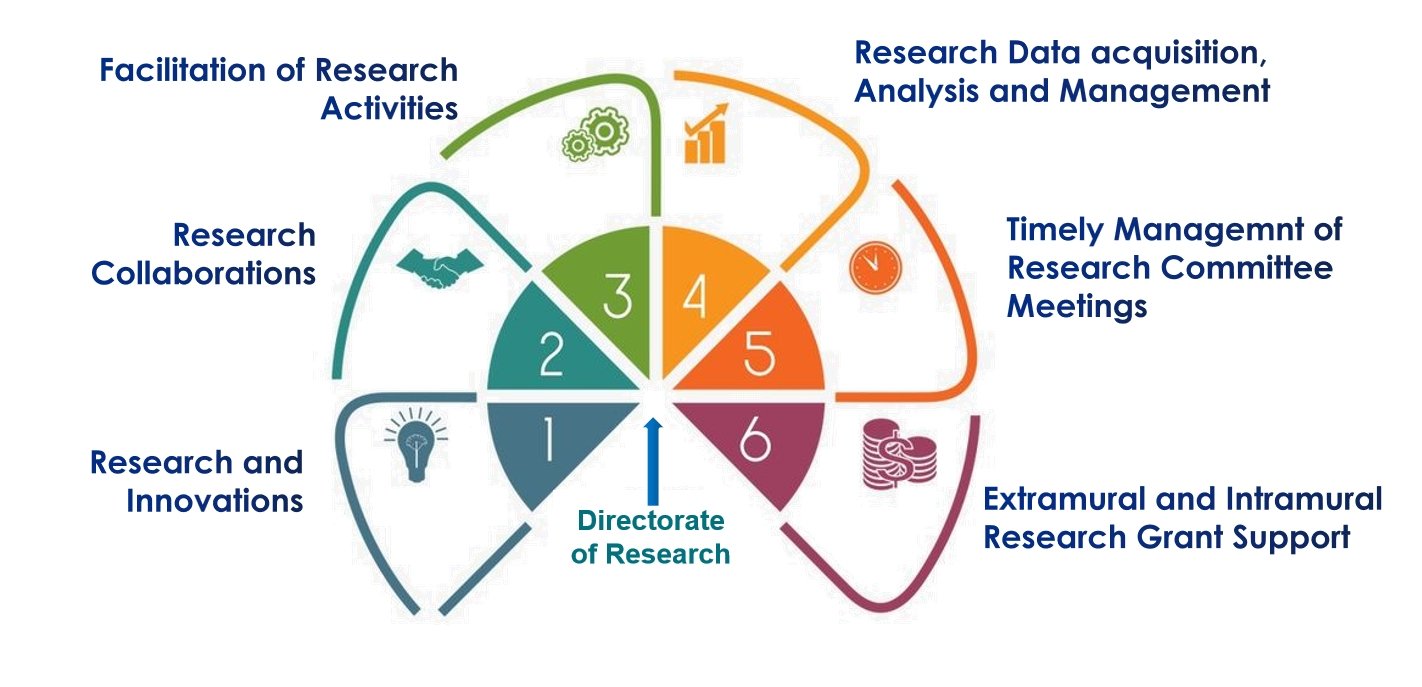
Directorate of Research and Innovation
Contact:
Vision
Mission
- To empower and support faculty, students, and staff to conduct world-class research across disciplines.
- To foster a collaborative research environment that promotes interdisciplinary exploration and knowledge creation.
- To secure funding and resources to advance research excellence and societal impact.
- To disseminate research findings through effective communication and knowledge exchange because of translational research.
- To attract and retain top research talent to strengthen the university's global reputation.
Objectives
- To conduct Training workshops (Research Methodology, IPR, GCP, GCLP)
- To undertake multiple scrutiny of all research proposals
- To obtain approval from IEC for all research projects
- To sanction funds for implementation of research proposals
- To monitor the quality of research work continuously
- To encourage researchers to obtain funds from external agencies
- To increase publications in the reputed, indexed journals
- To encourage & guide staff to apply for patents & copy rights
- To encourage learners & staff to organize & participate in Conferences & Workshops
- To encourage staff to write books & book chapters
- To publish quarterly Journal of Krishna Vishwa Vidyapeeth "Deemed To Be University"
About the Directorate of Research
The Directorate of Research at Krishna Vishwa Vidyapeeth (Deemed to be University) (KVV) is dedicated to advancing the fields of medical, paramedical, pharmaceuticals, and allied sciences and technology. The Directorate is established to enhance research and innovation activities by facilitating students, researchers, and teachers through a comprehensive and interdisciplinary approach to improving research outcomes for the benefit of the broader community. KVV stands as a beacon of research and innovation with several fully operational and dedicated units under the Directorate of Research, including:
- Hon'ble Late Shree Jayawantrao Bhosale Innovation and Incubation Centre (JBIIC)
- Centre for Research Innovation and Commercialization (CRIC), a joint centre of KVV and SPPU-Research Park Foundation at SPPU, Pune
- Specialized Research Laboratories
These units serve as hubs for cutting-edge research activities across various domains, including medicine, dental, physiotherapy, nursing, paramedical, pharmaceuticals, and allied sciences and technology. They focus on conducting fundamental and translational research to develop innovative solutions addressing real-time problems in the healthcare sector. With national and international research collaborations and a robust network of industry partners, healthcare centers, and end-users, KVV is well-equipped to understand and meet the needs of the healthcare community.


Thrust Areas of Research:
KVV also boasts a dedicated NABL-accredited diagnostic center, Molecular Biology and Genetics Laboratory, Lead Referral Laboratory, Toxicology Laboratory, Cancer Research Institute, Academic Institutes running over 70 different programs, and a 1240-bedded hospital, all actively engaged in research and innovation activities. For over three decades, KVV has been involved in fundamental, applied, and translational research in various fields, including:
| Cancer Biology | Physiotherapy |
| Infectious Diseases (TB, Malaria, AIDS) | Pharmaceuticals |
| Neuroscience | Medical Biotechnology |
| Cardiovascular Diseases | Microbiology |
| Diabetes | Molecular Biology |
| Mother and Child Nutrition | Genetics |
| Nutrigenomics | Biomedical Engineering and Technology |
| Dental Science |
KVV conducts extensive interdisciplinary translational research activities in the departments of medicine, physiotherapy, dentistry, nursing, pharmacy, and biotechnology to meet the needs of healthcare professionals and society. The researchers at KVV are keen to contribute to areas such as:
- Non-invasive cancer diagnosis
- Targeted drug delivery techniques
- Early diagnosis and treatment of infectious diseases like TB, Malaria, and VVC
- AI and ML interventions in healthcare
- Drug discovery and development
- Integrative and evidence-based statistical studies on community medicine
- Synthesis and formulations of compounds of medicinal importance
- Nanosensors and nanomedicine
- Genetic studies of diseases and disorders
- Molecular diagnosis of diseases
- Non-communicable diseases like hypertension and diabetes
Directorate of Research and Innovation Staff

Dr. (Brig.) G. Himashree
M.D. (Physiology)
Director of Research
(Extension - 460)
Email ID: director.research@kvv.edu.in

Dr. D. K. Agarwal, PhD
Director, Innovation,Incubation and Entrepreneurship (IIE)
(Extension - 452)
Email ID: director.iie@kvv.edu.in

Dr. S. V. Kakade, PhD
Assist. Director Research


Dr. R. S. Phatak, PhD
Assistant professor and Assistant Editor, JKIMSU
LinkedIn ProfileORCiD ID: 0000-0001-7900-0633



Dr. Mrs. T. S. Bhosale, Ph.D.
Statistician

Dr. D. A. Mane, Ph.D.
Statistician

Dr. M. M. Alate, Ph.D.
Statistician

Smt. Nandinee Chavan
Clerk

Mrs. Pranita Kadam
Clerk

Mr. D. M. Pawar
Office Attendant
Research collaborations
Research outcomes
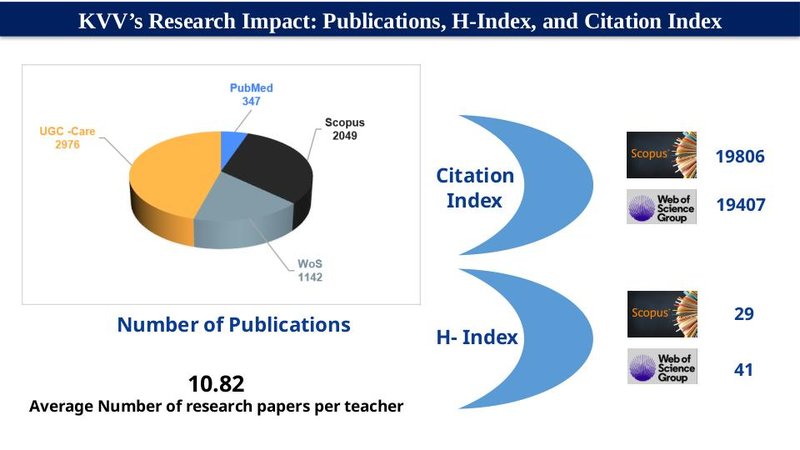
PUBLICATIONS
Number of research papers in the approved list of Journals included in Scopus/Web of Science/PubMed (2015-2020)
| Calendar Year | Scopus | Web of Science | PubMed | UGC listed |
|---|---|---|---|---|
| 2020 | 486 | 97 | 58 | 533 |
| 2021 | 79 | 95 | 31 | 150 |
| 2022 | 341 | 240 | 32 | 605 |
| 2023 | 660 | 318 | 48 | 860 |
| 2024 | 714 | 324 | 155 | 501 |
| 2025 | 50 | 13 | 30 | 72 |
| Total | 2330 | 1887 | 354 | 2721 |
Number of research papers in the approved list of Journals notified on UGC website (2015-2020)
| Calendar Years | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | Total |
|---|---|---|---|---|---|---|---|
| Number of research papers | 590 | 234 | 605 | 861 | 1002 | 72 | 3364 |
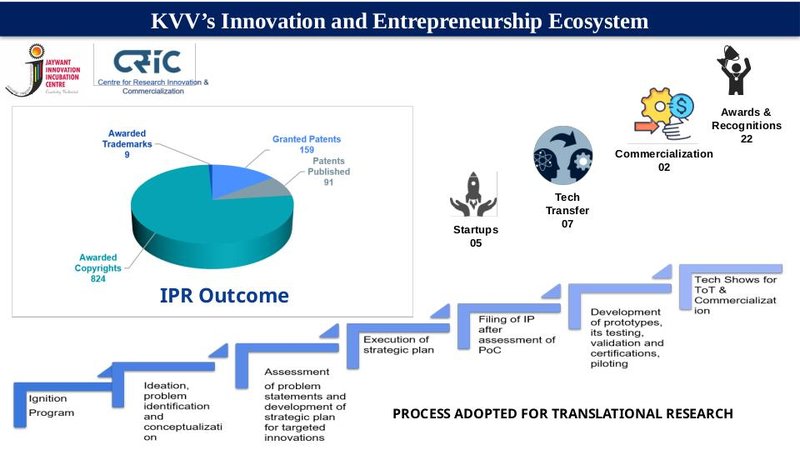
Total number of Patents/ Copyrights published/awarded/technology-transferred
| 2020-2021 | 2021-2022 | 2022-2023 | 2023-2024 | 2024-2025 | Total | |
|---|---|---|---|---|---|---|
| Patent and Design Granted | 15 | 27 | 47 | 80 | 42 | 211 |
| Patent Published | 20 | 24 | 16 | 12 | 12 | 84 |
| Copyright Awarded | 246 | 93 | 118 | 274 | 248 | 979 |
| Technology-transfer | 2 | 2 | 0 | 2 | 1 | 7 |
| Total | 283 | 146 | 181 | 368 | 303 | 1281 |
Total number of books/chapters in edited volumes and papers in National/International conference-proceedings published
| Calendar Years | 2020-2021 | 2021-2022 | 2022-2023 | 2023-2024 | 2024-2025 | Total |
|---|---|---|---|---|---|---|
| Number of books/ chapters in edited volumes and papers in National/International conference-proceedings published per teacher and indexed in Scopus/Web of Science/ PubMed UGC-CARE list during the year | 128 | 12 | 33 | 14 | 122 | 309 |
Hon'ble Shree Jayawantrao Bhosale Innovation and Incubation Center
ABOUT THE CENTRE
Hon’ble Shree Jaywantrao Bhosale Incubation and Innovation Center (JBIIC) is an interdisciplinary platform that creates a safe anchorage for novel ideas in the domain of medicine and allied sciences. With opportunities for individual researchers and group collaboration across all the disciplines of medicine, allied sciences, engineering, and technology, it’s a place that fosters a culture of innovation through the ideation, creation, testing, and validation of research concepts by developing prototypes for the growth of healthcare sector. This will establish a strong link between academics, industries, and end-users.